Product
rating
description
Collection
availability
Product Code
SKU
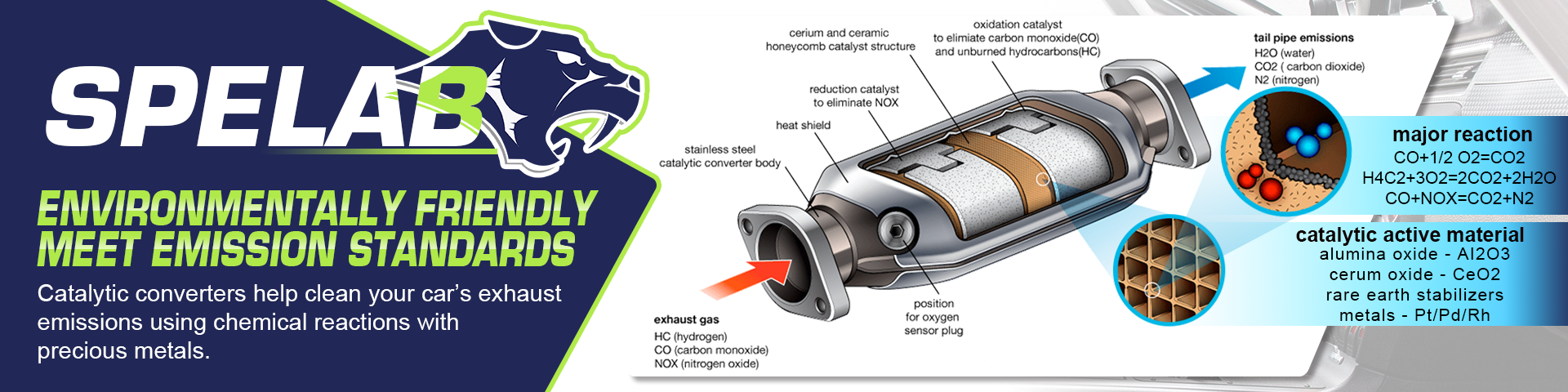
Catalytic converters change harmful substances in a car's exhaust gasses, such as carbon monoxide, nitric oxide, nitrogen dioxide and hydrocarbons, into less harmful substances like carbon dioxide and water vapour by means of chemical reactions.
The interior of the "cat" is usually filled with a honeycomb structure onto which a coating is applied that contains a catalyst - the substance that creates a reaction with the exhaust gasses, changing their chemical structure. Precious metals like palladium, rhodium and platinum are commonly used as the catalyst.
There are various types of catalytic converter. A simple "two-way" oxidation cat works to turn carbon monoxide (CO) to carbon dioxide (CO2) and hydrocarbons, which are basically particles of unburnt fuel, to carbon dioxide and water. More advanced "three-way" catalytic converters are fitted to modern cars and these do the above while also reducing emissions of nitric oxide (NO) and nitrogen dioxide (NO2) which together are more commonly known as NOx, a major cause of localised air pollution.

Rick Kment
Nice kit, with out the premium price. A must for power strokes. Comes from over seas. Took about 10 days to receive, with good Communication along the way. Going to consider other offerings from SPELAB.

Danny Pszenitzki
Great product easy install! Everything worked great have driven 10,000 miles pulling with product! No complaints.

Johnny Wireman
Arrived in a timely manner, installed about 2 hours , they sound amazing , I just wish there was a phone number to contact , some of us are not as knowledgeable as some when comes to computers lolo

OMAR SILVA-CHARBONIER
Good material, good prices, Only the flanges need to adapt, perfect fit just need modifications in the connection to the pipes, different angle 📐 flange need more than 90 degrees to fit the pipes , other than that is perfect fit in the engine M54, my chassis is E46 thanks for the product







